Description
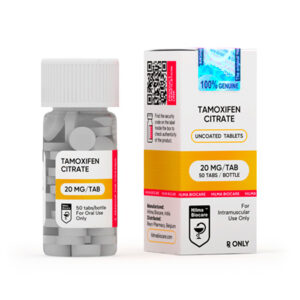
Tamoxifene Citrate
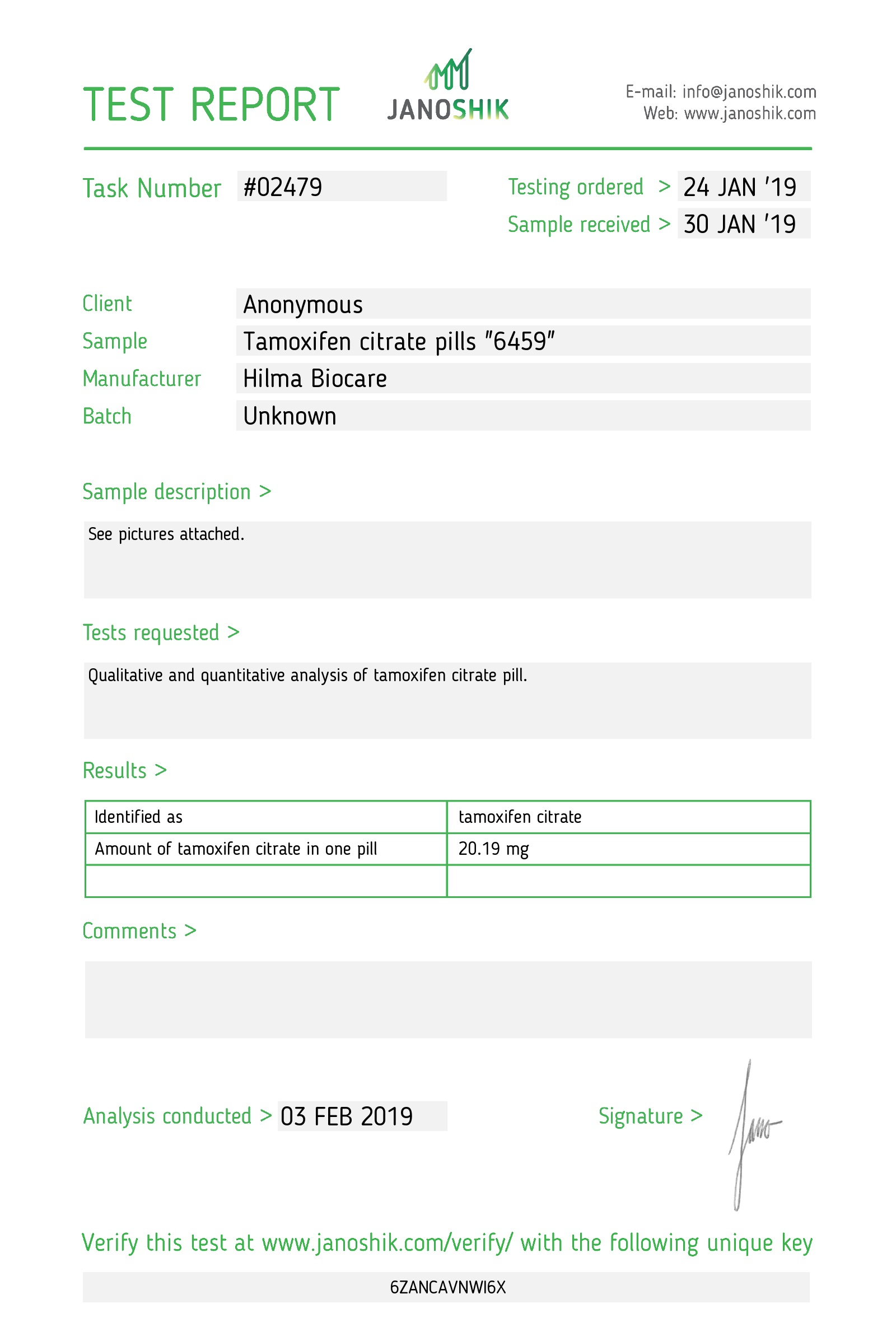
Strength: 20 mg
Molecular Formula: C26H29NO
Molecular Weight: 371.51456 g/mol
Active Ingredient: Tamoxifen citrate
CAS number: 54965-24-1
Dosage Form: Tablet
Route: Oral
Market Status: Prescription
Company: Hilma Biocare
DESCRIPTION
Tamoxifen citrate 20 is a nonsteroidal agent that has demonstrated potent antiestrogenic
effects related to its ability to compete with estrogen for binding sites in target tissues.
Tamoxifen citrate is an oral anti- estrogen and estrogen antagonist which competes with
estrogen at the receptor sites reducing estrogenic expression. Tamoxifen citrate is a
Selective Estrogen Receptor Modular (SERM) acting via estrogen site competition as
opposed to Aromatase Inhibitor (Al) drugs which prevent aromatase derived estrogens from
being created.
CLINICAL PHARMACOLOGY
The antiestrogenic effects of tamoxifen citrate may be related to its ability to compete with
estrogen for binding sites in target tissues such as breast. Tamoxifen citrate inhibits the
induction of rat mammary carcinoma induced by dimethylbenzanthracene (DMBÁ) and
causes the regression of already established DMBA- induced tumors. In this rat model,
tamoxifen appears to exert its antitumor effects by binding the estrogen receptors. In
cytosols derived from human breast adenocarcinomas, tamoxifen competes with estradiol
for estrogen receptor protein. Following a single oral dose of 20 mg tamoxifen citrate, an
average peak plasma concentration occurred approximately 5 hours after dosing. The
decline in plasma concentrations of tamoxifen citrate is biphasic with a terminal elimination
half-life of about 5 to 7 days. After initiation of therapy, steady state concentrations for
tamoxifen citrate are achieved in about 4 weeks and steady-state concentrations for
N-desmethyl tamoxifen citrate are achieved in about 8 weeks, suggesting a half- life of
approximately 14 days for this metabolite. Tamoxifen citrate is extensively metabolized after
oral administration. N-desmethyl tamoxifen is the major metabolite. Other minor metabolites
have been identified. Studies in women receiving 20 mg of 14C radio-IO labeled tamoxifen
citrate have shown that approximately 65% of the administered dose was excreted from the
body over a period of 2 weeks.
INDICATION AND USAGE
Oncology: For adjuvant treatment of breast cañcer, treatment of axillary nodenegative breast
cancer, treatment of ductal carcinoma in situ, and reduction of breast cancer in high risk
women. Tamoxifen citrate is frequently used as an antiestrogen in the treatment of
metastatic mammary carcinoma, especially in post- menopausal women, and patients with
endometrial cancer, renal cancer, soft tissues sarcoma, and pituitary tumors.
Fertility: For treatment of infertility in women with anovulatory disorders and oligospermia in
men.
Male Elevated Estrogen: For use as an antiestrogen to reduce undesirable estrogen-linked
expression in males with elevated estrogen levels either naturally or due to androgen
therapy.
Testosterone Suppressed Males: For use in males with suppressed endogenous
testosterone production due to androgen therapy after exogenous androgens have cleared.
CONTRAINDICATIONS
Patients with known hypersensitivity to the drug or any of its ingredients. Patients who
require concomitant anticoagulant therapy. Patients with a history of deep vein thrombosis,
stroke, uterine malignancies, or pulmonary embolism. Patients with a history of ocular
disturbances, cataracts, or retinopathy. Patients with hypercalcaemia.
WARNINGS
To be used only under the care of a physician.
PRECAUTIONS
For women: Tamoxifen citrate may increase fertility. Tamoxifen citrate may cause fetal harm.
Tamoxifen citrate has been linked to severe liver and thromboembolic events. Monitor
patients CBC and liver function tests.
DRUG INTERACTIONS
Tamoxifen citrate 10 when used in combination with coumarin-type anticoagulants may
create an anticoagulant effect. Careful
monitoring of the patient’s prothrombin time is recommended Concomitant administration of
letrozole, anastrozole, phenobarbital, aminoglutethimide, or corticosteroids may result in
decreased tamoxifen serum levels Bromocriptine has been shown to elevate tamoxifen
concentration in serum.
ADVERSE REACTIONS
Most side effect studies are based on female cancer patients whose primary sex hormone
estrogen is effectively blocked through the use of tamoxifen citrate. Male patients have
demonstrated reduced levels of side effects. Side effects may include nausea, coughing,
vomiting and hot flashes, difficulty breathing; swelling of your face, lips, tongue, or throat.
Sudden numbness or weakness, especially on one side of the body, sudden headache,
confusion, problems with vision, speech, or balance; chest pain, shortness of breath.
Reduced cognition and memory impairment, vaginal bleeding, amenorrhea, altered menses,
discharge, ovarian cysts or pelvic pain. Corneal changes, reduced color vision, cataract
formation, retinal dvt, and retinopathy. Thromboembolic events, anorexia, pain, depression.
Rarely elevation of liver enzymes and liver disease. Reduced platelet production,
leukopenia, thrombocytopenia. hypercalcaemia, peripheral oedema, distaste for food,
pruritus vulvae, depression, dizziness, light-headedness, headache, hair thinning, hair loss,
and vaginal dryness. Increases in serum triglycerides.Stop using tamoxifen and call your
doctor at once if you experience any of these serious side effects.
DOSAGE AND ADMINISTRATION
Adult male estrogen management: 10 – 20mg taken orally every other day. Adult male
gynecomastia onset: 40mg taken orally for 3 days. Reduce dosing thereafter. Endogenous
testosterone restart post-androgen therapy: 20mg per day taken orally for 10 to 14 days.
Adult male fertility: pursuant to advice of qualified physicians. Adult carcinoma: pursuant to
advice of qualified physician.
PRESENTATION
Tamoxifen citrate 20 mg uncoated tablets: 50 tablets in 1 bottle.
STORAGE
Store in a cool dry place between 15- 25°C. Protect from light.

Reviews
There are no reviews yet.