Description
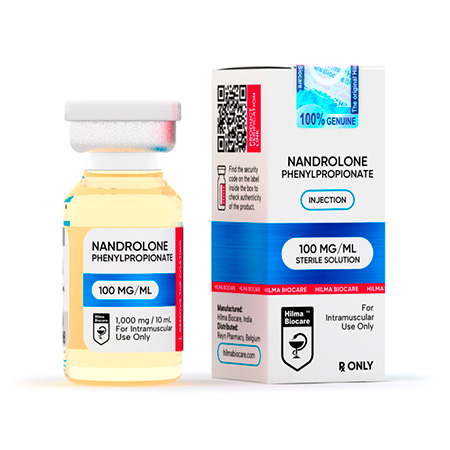
Nandrolone Phenylpropionate
Strength: 100 mg/ml
Molecular Formula: C27H34O3
Molecular Weight: 406.55706 g/mol
Active Ingredient: Nandrolone phenylpropionate
CAS number: 62-90-8
Dosage Form: Injeciable, oil base sterile solution
Route: Injection
Market Status: Prescription
Company: Hilma Biocare
DESCRIPTION
Nandrolone Phenylpropionate 100 are injectable anabolic preparations containing the
short-acting nandrolone phenylpropionate ester. Serum nandrolone levels nel rapidly
increase after IM administration with peak serum levels being reached within 24 to 36 hours.
NPP clears rapidly avoiding the retention issues associated with the traditional nandrolone
decanoate. Nandrolone phenylpropionate accelerates muscle growth, stimulates appetite,
increases red blood cell production, and improves bone density.
INDICATIONS
Osteoporosis due to androgen deficiency in hypogonadal males. Treatment of anemia in
cases of renal insufficiency where oxymetholone is contraindicated. Restoration of muscle
mass in patients with muscular atrophy after traumatic recovery.
ADVERSE REACTIONS
Male: Gynecomastia, excessive frequency and duration of penile erections, oligospermia.
Skin and Appendages: Hirsutism, male pattern baldness and acne, gynecomastia.
Fluid/electrolyte Disturbances: Retention of sodium, chloride, water, potassium, calcium, and
inorganic phosphates.
Gastrointestinal: Nausea, cholestatic jaundice, alterations in liver function tests; rarely,
hepatocellular neoplasms, peliosis hepatitis, hepatic adenomas, and cholestatic hepatitis.
Hematologic: Suppression of clotting factors II, V, VII, & X; bleeding in patients on
anticoagulant therapy.
Nervous System: Increased or decreased libido, headache, anxiety, depression, and
generalized paresthesia.
Other: Serum lipid changes, hypercalcaemia, hypertension, oedema, priapism, and
potentiation of sleep apnea.
CONTRAINDICATIONS
Not for use in women or children. Patients with known hypersensitivity to any ingredients in
this product. Patients with known or suspected prostatic, testicular, hepatic, or mammary
carcinoma. Patients with nephrosis or the nephrotic phase of nephritis, hypercalcaemia,
oedema, jaundice, or liver or kidney disease with impaired bilirubin excretion. Products
containing androgens should not be used in women as they may cause virilization and fetal
harm. Latent or overt cardiac failure, renal dysfunction, hypertension, epilepsy or migraine or
a history of these conditions, since anabolic steroids may induce salt and fluid retention.
PRECAUTIONS
Because androgens may alter serum cholesterol concentration, caution should be used
when administering these drugs to patients with a history of myocardial infarction or coronary
artery disease. Patients on oral anticoagulant therapy require close monitoring especially
when androgens are started or stopped.
Diabetics: androgens may alter the metabolism of oral hypoglycemic agents or may change
insulin sensitivity in patients with diabetes mellitus which may require adjustment of dosage
of insulin and other hypoglycemic drugs.
DRUG INTERACTIONS
Oral hypoglycemic agents: may inhibit the metabolism of oral hypoglycemic agents which
may require adjustment of dosage. Anticoagulants: Patients on anticoagulants should be
carefully monitored during anabolic steroid therapy as anabolic steroids may increase
sensitivity to oral anticoagulants. Patients should be monitored regularly during anabolic
steroid therapy, particularly during initiation and termination of therapy.
PATIENT MONITORING
Serum Cholesterol, HDL, LDL. TG. Hemoglobin and Hematocrit, Hepatic function tests –
AST/ALT, Prostatic specific antigen PSA, Testosterone: total, free, and bioavailable.
Dihydrotestosterone & Estradiol. alumo Male patients over 40 should undergo a digital rectal
examination and evaluate PSA prior to androgen use. Periodic evaluations of the prostate
should continue while on androgen therapy, especially in patients with difficulty in urination
or with changes in voiding habits.
DOSAGE AND ADMINISTRATION
Adult male: 100 – 150 mg injected IM every 3 – 5 days for a duration of 8 to 12 weeks.
PRESENTATION
Nandrolone Phenylpropionate 100 mg/ml, 10 ml multiple dose vial.
STORAGE
Store in a cool dry place between 15- 25°C. Protect from light.


Reviews
There are no reviews yet.