Description
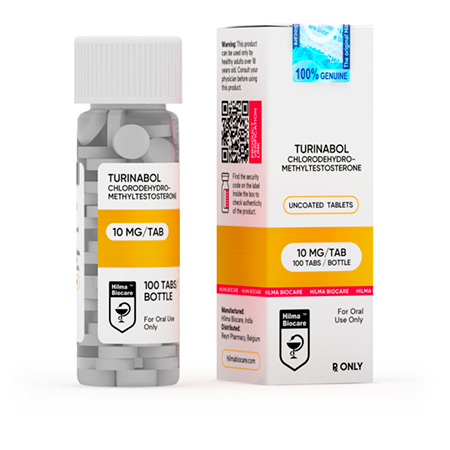
Turinabol
Strength: 10 mg
Molecular Formula: C20H27CLO2
Molecular Weight: 334.88018 g/mol
Active Ingredient: Chlorodehydromethyltestosterone
CAS number: 2446-23-3
Dosage Form: Tablet
Route: Oral
Market Status: Prescription
Company: Hilma Biocare
DESCRIPTION
Turinabol 10 is a 17-alpha alkylated anabolic steroid for oral use. It promotes anabolism
through androgen receptor activity and is strongly anabolic and moderately androgenic.
When taken in clinical doses, it produces potent increases in strength and moderate
increases in muscle mass. In short term clinical trials, it has been well tolerated in healthy
male subjects during 6 weeks of treatment.
CLINICAL PHARMACOLOGY
Anabolic steroids are synthetic derivatives of testosterone. Certain clinical effects and
adverse reactions demonstrate the androgenic properties of these drugs. Anabolic steroids
suppress the gonadotropic functions of the pituitary and may exert a direct effect upon the
testes. During exogenous administration of anabolic androgens, endogenous testosterone
release is inhibited through inhibition of pituitary luteinizing hormone (LH). At larger doses,
spermatogenesis may be suppressed through feedback inhibition of pituitary follicle
stimulating hormone (FSH) In a single dose pharmacokinetic study of Chlorodehydromethyltestosterone, 10mg administered orally to healthy males reached maximum serum levels in
3 hours It is subject to first pass hepatic deactivation rapidly with a strong dominance of
bioactive metabolites over the parent drug within a few hours. Prior to reaching serum orally
dosed subjects exhibited significant variations in serum absorption levels linked to variations
in enterohepatic circulation as demonstrated by radiographic label tracing. In a study
involving IV administration of Turnibol 10, the terminal half-life of the parent molecule was
found to be 16-hours. Given the lack of consensus on the bioactivity of its metabolites and
the predominance of metabolites over the parent drug after oral administration a clinically
useful biological half-life remains unclear. Turnibol has maxim demonstrated an affinity for
SHBG introducing competition for testosterone binding, thereby yielding increased plasma
levels of unbound testosterone.
INDICATION AND USAGE
Turnibol 10 is indicated as alternate or adjunctive therapy in patients for the promotion of
weight gain following weight loss and/or muscular atrophy associated with extensive surgery,
chronic infections, Jong term hospitalization, or severe trauma. Turnibol 10 is indicated to
compensate for protein catabolism consequent to corticosteroid therapy.The physician and
patient must consider the risks of therapy versus the potential benefits.
CONTRAINDICATIONS
Patients with known hypersensitivity to any ingredients in this product. Patients with known
or suspected carcinomas of the breast, testis, or prostate. Patients with severe heart
disease, liver disease, or kidney disease or with a history of epilepsy. Products containing
androgens should not be used in women as they may cause virilization and fetal harm.
Patients with nephrosis or the nephrotic phase of nephritis, ed Patients with hypercalcemia.
sleM Patients with pre-existing cardiac, renal, and/or hepatic disease.
PRECAUTIONS
Elevated liver enzymes and in rare cases hepatic liver dysfunction may occur. Periodic liver
function tests should be conducted given the association of 17-alpha- alkylated androgens
with hepatotoxicity and treatment discontinued if the patient presents with signs of
hepatotoxicity or jaundicing. Edema may be increased in patients on concurrent adrenal
cortical steroid or ACTH therapy. Androgenic anabolic steroids have been associated with
changes in serum lipids, generally with decreases in high-density lipoprotein (HDL)
concentration and increases in low-density lipoprotein (LDL) concentration, a profile known
to be associated with increased risk of atherosclerosis and associated risk of coronary artery
disease. Thus caution should be used when administering these drugs to patients with a
history of myocardial infarction or coronary artery disease. Anabolic steroids may reduce
clotting factors II, V, VII, and X, and may increase prothrombin time (PT). Patients should be
instructed to report any use of warfarin or other oral anticoagulant therapy and any irregular
bleeding. Male patients receiving androgenic anabolic steroid therapy may be at an
increased risk of prostate hypertrophy.
DRUG INTERACTIONS
Oral hypoglycemic agents: may inhibit the metabolism of these agents which may require
adjustment of dosage. Anticoagulants: Patients on anticoagulants should be carefully
monitored during anabolic steroid therapy as anabolic steroids may increase sensitivity to
oral anticoagulants. Patients should be monitored regularly during anabolic steroid therapy,
particularly during initiation and termination of therapy.
Diabetics: androgens may alter the metabolism of oral hypoglycemic agents or may change
insulin sensitivity inpatients with diabetes mellitus which may require adjustment of dosage
of insulin and other hypoglycemic drugs.
ADVERSE REACTIONS
Male: Gynecomastia, excessive frequency and duration of penile erections, priapism,
oligospermia.
Skin and Appendages: Hirsutism, male pattern baldness and acne, gynecomastia.
Fluid/electrolyte Disturbances: Retention of sodium, chloride, water, potassium, calcium, and
inorganic phosphates. Gastrointestinal: Nausea, cholestatic jaundice, alterations in liver
function tests; rarely, hepatocellular neoplasms, peliosis hepatitis, hepatic adenomas, and
cholestatic hepatitis. Hematologic: Suppression of clotting factors II, V, VII, & X bleeding in
patients on anti- coagulant therapy. Nervous System: Increased or decreased libido,
headache anxiety, depression, and generalized paresthesia. Metabolic: Reduced glucose
tolerance, changes in creatinine clearance. Other: Serum lipid changes, polycythemia,
hypercalcaemia, hypertension, and potentiation of sleep apnea.
PATIENT MONITORING
Serum Cholesterol, HDL, LDL, TG. Hemoglobin and Hematocrit, Hepatic function tests –
AST/ALT. Prostatic specific antigen – PSA, Testosterone: total, free, and bioavailable.
Dihydrotestosterone & Estradiol. valls Male patients over 40 should undergo a digital rectal
examination and evaluate PSA prior to androgen use. Periodic evaluations of the prostate
should continue while on androgen therapy, especially in patients with difficulty in urination or
with changes in voiding habits.
DOSAGE AND ADMINISTRATION
Adult males: 10-30mg taken orally per day in divided doses for a duration of 4 to 6 weeks.
PRESENTATION
Chlorodehydromethyltestosterone 10 mg uncoated tablets: 100 tablets in 1 bottle.
STORAGE
Store in a cool dry place between 15-25°C. Protect from light.